Demystifying PEM Membranes in Hydrogen Water
Share
Demystifying PEM Membrane: The Unsung Heroes of Hydrogen-Enriched Water
Hydrogen-enriched water is gaining attention for its potential health benefits, such as combating oxidative stress.But have you ever wondered how it’s made? It all starts with a scientific process called electrolysis, where water (H₂O) is split into hydrogen (H₂) and oxygen (O₂). At the heart of this process lies an ingenious innovation: the PEM membrane.
Without PEM, however, the system becomes prone to inefficiencies and potentially harmful byproducts. Here's a closer look at why PEM membranes matter and the risks of skipping them.
This blog breaks down what PEM membranes are, how they work, and why they matter for both technology and health.
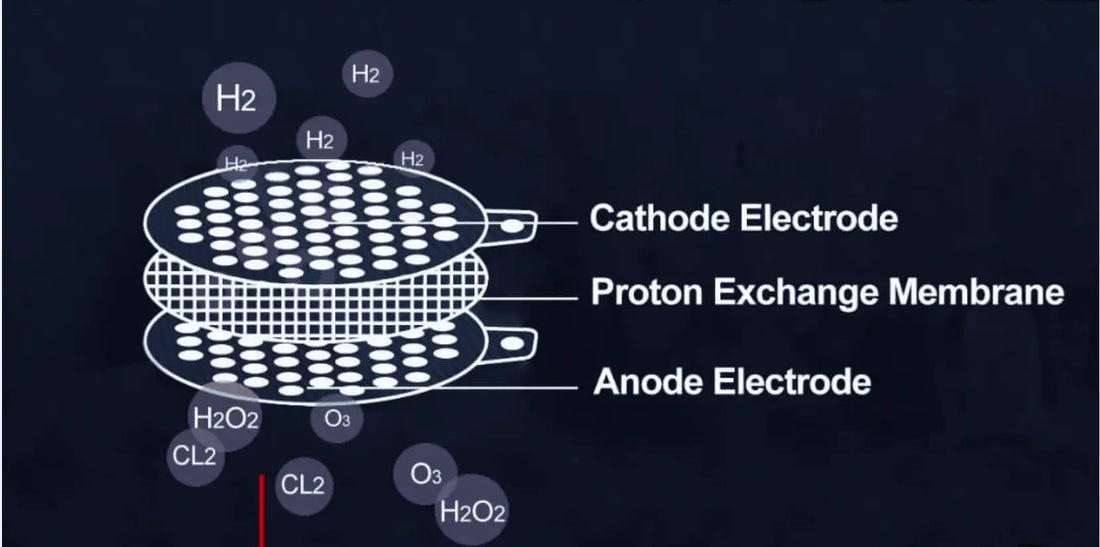
What Is a PEM Membrane?
A Proton Exchange Membrane (PEM), also known as a polymer electrolyte membrane, is a thin, flexible film with unique properties:
- Semipermeable: Allows specific particles (like protons) to pass through while blocking others.
- Proton Conductor: Facilitates the smooth movement of positively charged hydrogen ions (H⁺).
- Electrical Insulator: Prevents electrons from bypassing the external circuit.
- Chemical Separator: Ensures oxygen and hydrogen are isolated from one another during the reaction.
This selective filtering makes PEM membranes essential for clean, safe hydrogen-enriched water production.
How Does a PEM Membrane Work in Electrolysis?
Electrolysis is the backbone of hydrogen water production, and PEM membranes play a pivotal role in making the process efficient. Let’s simplify the steps:
The Gatekeeper
Instead of a liquid electrolyte, the system uses a solid PEM membrane. This film acts as a barrier, allowing only protons (H⁺) to pass while blocking oxygen and electrons.
Splitting Water
Inside an electrolyzer, water is split into hydrogen and oxygen using electricity. At the anode (positive side), water molecules are broken down into:
- Protons (H⁺): Positively charged hydrogen ions.
- Oxygen gas (O₂): Released into the air.
- Electrons (e⁻): Free to flow through an external circuit.
Proton Pathway
The PEM membrane acts as a selective highway for protons, allowing them to cross to the cathode (negative side). Meanwhile, oxygen stays behind, and electrons are directed along an external circuit.
Reuniting Protons and Electrons
At the cathode, protons combine with the electrons from the circuit to form pure hydrogen gas (H₂). This hydrogen is then dissolved into water, creating the hydrogen-enriched water we love.
What Happens When SPE Is Used Without PEM?
In some electrolysis systems, SPE (Solid Polymer Electrolyte) can be used as a standalone component without the addition of a PEM membrane. While SPE enables electrolysis, its absence of a dedicated proton exchange layer can lead to significant drawbacks:
1. Impurities in the Water
Without the separation provided by a PEM membrane, the process can allow small amounts of:
- Residual oxygen gas (O₂): This can remain dissolved in the water, altering its intended composition.
- Electrolyte contamination: Byproducts from the electrodes, such as trace metals or impurities, may leach into the water during the reaction.
These impurities can affect the water’s taste, odor, and overall safety.
2. Generation of Harmful Byproducts
Without the precise separation of PEM, unwanted side reactions may occur, leading to:
- Chlorine (Cl₂) or hypochlorite (OCl⁻): If tap water contains chloride ions (common in municipal water supplies), electrolysis without a PEM can produce chlorine gas or hypochlorite byproducts, which are harmful if consumed in excess.
- Peroxide formation: Uncontrolled reactions may lead to hydrogen peroxide (H₂O₂) creation, which is not safe for regular consumption.
3. Inefficient Hydrogen Production
The lack of a PEM can also disrupt the flow of protons and electrons, leading to:
- Lower hydrogen yield.
- Increased energy consumption.
- A higher likelihood of recombination, where separated hydrogen and oxygen molecules reform water instead of producing usable hydrogen gas.
PEM Membranes: The Quality Guarantee
When a PEM membrane is included in the electrolysis process, it ensures:
- Purity: Only protons pass through, while oxygen, electrons, and other impurities remain separated.
- Safety: No harmful byproducts like chlorine or hydrogen peroxide are formed, even when using regular tap water.
- Efficiency: Protons and electrons are routed correctly, maximizing hydrogen production and minimizing energy waste.
This makes PEM membranes the gold standard for producing high-quality hydrogen-enriched water that is clean, safe, and effective.
The Bigger Picture: Why PEM Matters
PEM membranes ensure the electrolysis process runs smoothly by maintaining separation between the chemical reactions. Without them, the separation of hydrogen and oxygen would be inefficient, leading to water without the elevated hydrogen levels necessary for its antioxidant benefits.
By enabling the creation of hydrogen-enriched water, PEM membranes make it possible to enjoy the potential health advantages of higher hydrogen concentrations, such as combating oxidative stress and promoting wellness.
In hydrogen-enriched water production, the presence of a PEM membrane isn’t just a technical detail—it’s a safeguard for water quality. Here's the breakdown:
- With PEM: Clean hydrogen gas is produced and dissolved into the water, free from harmful byproducts or contaminants.
- Without PEM: Electrolysis risks introducing impurities, undesirable tastes, and potential health risks.
By prioritizing systems with PEM technology, you ensure that your hydrogen-enriched water delivers all the benefits without compromising on safety or quality.
Simplified Science, Real Impact
In summary, PEM membranes aren’t just a component—they’re the backbone of safe and effective hydrogen-enriched water production. They:
- A PEM (Proton Exchange Membrane) is a specialized thin film that conducts protons and acts as a separator in the electrolysis process.
- Enable precise water splitting by separating protons, electrons, and oxygen.
- This membrane facilitates the production of hydrogen-enriched water by selectively allowing protons to pass through while keeping oxygen and electrons on separate paths.
- Prevent the formation of unwanted byproducts like chlorine or hydrogen peroxide.
- Without PEM membranes, electrolysis processes may introduce impurities and byproducts into the water, compromising its quality and safety.
- Deliver clean, pure hydrogen for healthier hydration.
Ready to see PEM technology in action? Explore our hydrogen products, equipped with PEM membranes to elevate your hydration experience today.
Cheers to innovation—and your health!
